Abstract
Introduction: Salvage autologous transplantation (sASCT) in multiple myeloma has been shown to induce superior durability of responses over low-dose alkylating consolidation therapy in the relapsed setting1. The optimum re-induction regimen leading to sASCT is yet to be defined though a combination of a proteasome inhibitor (PI) and immunomodulatory agent (IMiD) is likely to offer the best depth of response. UK-MRA Myeloma XII (ACCoRD study) aims to address the role of conditioning augmentation and post-transplant maintenance. As part of this study, a novel re-induction regimen was used, the results of an interim analysis (IA) of efficacy and safety are presented here.
Patients and Methods: The ACCoRd study enrolled patients who relapsed requiring treatment more than 12 months after a previous ASCT (ASCT1), delivering novel re-induction therapy with ixazomib, thalidomide and dexamethasone (ITD) prior to being randomized between a conventional high-dose melphalan (HDM) sASCT versus an ixazomib-augmented (iMel) sASCT. A second randomization between observation and post-transplant ITD consolidation with ixazomib maintenance, was conducted at D+100 post sASCT. All patients underwent genetic risk categorization by a central laboratory. High-risk genetic lesions were defined as t(4;14), t(14;16), t(14;20), del(17p) or gain(1q). On an intent-to-treat (ITT) basis, responses were assessed in accordance with the IMWG criteria with MRD defined by the limit of the multi-parameter flow assay (<10-5). We sought to define the overall response rate (ORR) to ITD re-induction and in a pre-planned sub-group analysis, compare ORR by relapse category (biochemical vs. clinical relapse), genetic risk status (standard risk, high risk [1 lesion, HiR] and ultra-high risk [≥2 lesions, UHiR]) at the time of trial entry and previous treatment received (PI exposure).
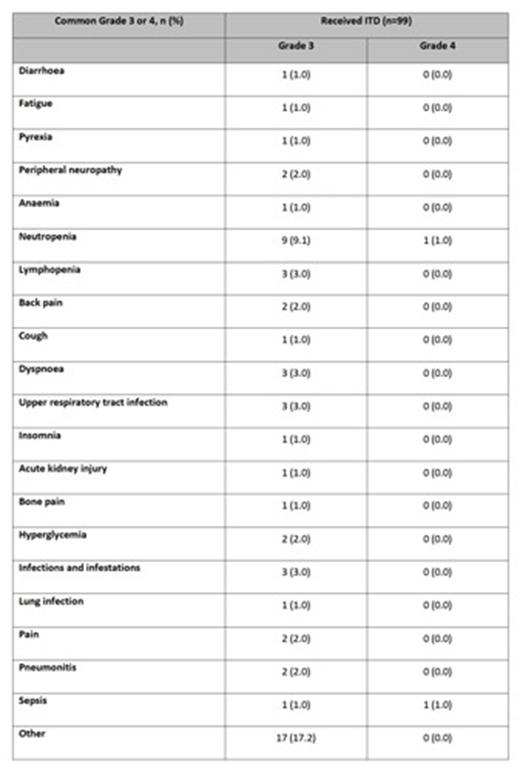
Results: To date, 206 patients have been recruited to the study, and 101 patients have completed re-induction. Median age was 62 (range 34, 73) with 11% of patients >70 years. The median observed TTP from ASCT1 was 31.5 months (range 9.8, 118.3). 15.8% of patients were deemed to have clinical relapse at trial entry based on CRAB criteria (anaemia 13.9%, renal disease 3.0% and hypercalcaemia 0.0%) and no significant difference in ASCT1 TTP was evident between clinical and biochemical relapse groups (21.8m vs 31.9m; Wilcoxon p=0.2201). 68.2% had standard risk disease, 24.2% had HR disease and 6.1% had UHiR disease at trial entry, with gain(1q) (25.8%) being the commonest abnormality at relapse. 62.5% had standard risk disease, 27.5% had HR disease and 10% had UHiR disease at diagnosis with gain(1q) (20.8%) being the commonest abnormality. The ORR was 66.3%, with ³VGPR in 26.7%. The ORR and ³VGPR did not differ between biochemical vs. clinical relapse (ORR 56.3% vs 68.3%; ³VGPR 12.5% vs 29.3%; P=0.5585), genetic risk status (SR/HiR: ORR 57.8% vs 50.0%; ³VGPR 20% vs 31.3%; P=0.5585) but prior PI exposure was important (exposed vs naive: ORR 53.5% vs 75.9%; ³VGPR 16.3% vs 34.5%; P=0.0290). Progressive disease (PD) was recorded in 11.9%, with PD more commonly seen in PI-exposed (18.6%) and HiR (18.8%). 59/101 patients proceeded to transplant randomization so far, the main reasons for failing to proceed was PD (11.9%). 475 cycles of ITD were administered to 99 patients, with a median of 5 cycles per patient (range 1, 7). 41 SAEs were reported in 30 patients with 17 SAEs suspected related to trial medication, summarized in Table 1. 45.5% of patients had treatment modifications (14.9% with ixazomib, 37.6% with thalidomide, 24.8% dexamethasone) with treatment discontinuations for toxicity reported in 3.9% of patients. The commonest reasons for treatment discontinuation were maximum response (67.3%), PD (11.9%) and withdrawal (7.9%).
Conclusion: We demonstrate the efficacy and safety profile of the novel triplet combination of ixazomib, thalidomide and dexamethasone as a re-induction regimen suitable for patients progressing to a ASCT. Further data will be forthcoming as the trial continues to recruit towards target, with the true impact on depth of response pending interim analysis of the transplant randomization.
G Cook, et al. The Lancet Oncology, Vol. 15, No. 8, p874-885.
Cook:Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria; Glycomimetics: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau. Parrish:Jazz: Honoraria, Speakers Bureau; Janssen: Speakers Bureau; Celgene: Speakers Bureau. Yong:TAkeda: Honoraria, Research Funding; janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria, Research Funding. Cavenagh:Takeda: Research Funding, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Snowden:Jannssen/J&J: Other: Speaker fees; Jazz & Sanofi: Other: Speaker fees at ASH. Drayson:Abingdon Health: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Garg:Amgen: Honoraria, Other: Travel Support; Novartis: Other: travel support, Research Funding; Janssen: Honoraria; Takeda: Other: Travel Grant. Cairns:Merck Sharp and Dohme: Research Funding; Amgen: Research Funding; Celgene: Research Funding. Ashcroft:Janssen: Consultancy, Honoraria, Speakers Bureau; Celgene Corporation: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Speakers Bureau; Amaris Medical: Consultancy, Honoraria. Striha:MSD: Research Funding; Celgene: Research Funding; Janssen: Research Funding; Amgen: Research Funding; Abbvie: Research Funding. Hockaday:Janssen: Research Funding; Abbvie: Research Funding; Amgen: Research Funding; Celgene: Research Funding; Millenium: Research Funding; MSD: Research Funding. Owen:Janssen: Consultancy, Other: Travel support; Takeda: Honoraria, Other: Travel Support; Celgene: Consultancy, Honoraria, Research Funding. Williams:Amgen: Honoraria, Speakers Bureau; Takeda: Honoraria, Other: Travel support; Celgene: Honoraria, Other: Travel Support, Speakers Bureau; Janssen: Honoraria, Other: Travel support, Speakers Bureau. Cook:YAkeda: Honoraria; Jazz: Honoraria; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Chugai: Honoraria; Amgem: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal